Relative Mass
- Relative Mass
- Relative Mass And The Mole
- Relative Mass Of Subatomic Particles
- Relative Mass Of A Proton
Why is the mass number an integer but the relative atomic mass is not?
Relative atomic mass meaning: 1. The mass of an atom of a particular chemical element, usually expressed in atomic mass units 2. The relative mass of an object is the comparison of the mass of the object to the mass of a standard object.
Relative Mass
1 Answer
- Relative mass is an important concept in chemistry. It exists to simplify the process of working out the mass of an atom or molecule. In absolute units, protons and neutrons have masses on the order of 10 − 27 kilograms, which is a billionth of a billionth of a billionth of a kilogram, and electrons have even smaller mass of about 10 − 30 kilograms, about a thousand times less than a.
- Relative means in relation to, or in comparison to. So for example, if you are asked for a vehicle’s speed relative, or in relation to, the ground, it might be different from the same vehicle’s speed relative, or in relation to, another vehicle.
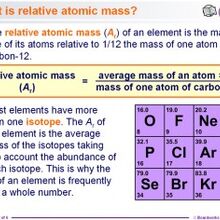
- The relative atomic mass of an element is a weighted average of the masses of the atoms of the isotopes - because if there is much more of one isotope then that will influence the average mass much.

Well the mass number refers to a given
Explanation:
Relative Mass And The Mole
A nuclide is a specific isotope, which of course has specific
On the other hand, the
By way of example, the element boron has
And so the
Relative Mass Of Subatomic Particles
Relative Mass Of A Proton
Related questions
